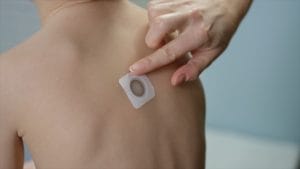
 Photo: DBV Technologies
Photo: DBV Technologies DBV Technologies, the French company that developed the Viaskin Peanut patch, announced in October 2017 that while 35.3 percent of patients responded to the immunotherapy treatment after one year, the difference between the treatment arm and the placebo arm of the study didn’t meet a statistical measure that was set out by the FDA.
The result is a disappointment to the allergy community, which has been waiting anxiously for an FDA-approved treatment for peanut allergies. But DBV says it still plans to apply for approval with the federal agency for its technology that allows small amounts of peanut protein to be absorbed through the skin to promote desensitization.
“There was contact between the FDA and DBV once that result was known and the FDA provided encouragement about proceeding as planned,” says Dr. Todd Green, vice president of medical affairs at DBV. “There is precedent with other drugs getting approved for other situations where they didn’t hit that number, but were close, and the drugs were still found to be safe and efficacious.”
Investors are less certain, however. DBV shares fell sharply the day the results were announced.
Response, Duration of Wearing
 Dr. David Fleischer: stats don't tell whole story.
Dr. David Fleischer: stats don't tell whole story. The 35 percent is also a bit misleading. While the placebo-controlled portion of the study is only one year, previous studies have shown that the patch is more effective the longer it’s worn. Results from a Phase 2 study first released in released in March and recently published in the Journal of the American Medical Association showed that 83 percent of kids aged 6 to 11 were able to tolerate 10 times the amount of peanut after three years on the patch.
“We need more than oral immunotherapy,” says Fleischer, who is an associate professor of pediatrics and allergy at the University of Colorado. “You have to balance out what’s going to work best for which patient.”
Previous studies of Viaskin, as well as studies looking at oral immunotherapy, have shown that the younger the patient is at the time of treatment, the better. Fleischer says the FDA required a certain number of 4- and 5-year-olds to be included in this study, and the results for those patients will be looked at closely.
Younger Kids Studied
Another Phase 3 trial in kids even younger, aged 1 to 3 years is underway, as is a Phase 3 study looking at using Viaskin Peanut in clinical practice, rather than a double-blind trial.
Dr. Robert Wood, chief of allergy and immunology at Johns Hopkins Children’s Center, one of the PEPITES study sites, is cautiously optimistic Viaskin will win FDA approval. “While I was hoping to see more conclusive results, I still believe that this treatment will prove to be worthwhile as additional studies progress,” he says.
Fleischer suggests people stay tuned for more information. “We just got the full dataset. We will analyze it and start writing and submit the final results to a journal.”





